Research & Discovery
Interrupting cancer’s cascade
Priscilla Hwang is unwinding how breast cancer spreads — and trying to shut it down
It’s rush hour right now in Priscilla Hwang’s lab. Actually, it’s rush hour almost all the time. Breast cancer cells roam this way and that, day and night, through the fluid-filled channels of tiny devices arranged inside incubators — the cells doing their damnedest to sow chaos, if only they could.
They’re not moving very quickly or very far. In a day, a clump of cells in these experiments might migrate 200 microns, the thickness of two sheets of paper. But they’re migrating. And were these cancer cells inside a body, rather than on a microscope slide, this would mark the moment they attempt to hop a lymphatic or blood-vessel highway to some other tissue, someplace to start a second tumor and tip this disease into stage 4.
For Hwang, Ph.D., an assistant professor in the College of Engineering and recent recipient of a prestigious National Science Foundation grant, unwinding the process of how these clusters of cells break away to spread is the first step in figuring out how to stop them.
“What kind of signals are the tumor cells receiving that turn on that migration pathway,” she says, “[that] tell them, ‘OK, now it’s time to go?’”
So far the answers she’s found have sharpened the picture, though they reveal a situation that’s even more complex than it seemed.
Take, for instance, the cells in charge of all this. Breast cancer cells often migrate in a cluster of a few hundred cells (rather than individually, as is common with some cancers), and scientists have learned over the past decade or so that group migration is marshaled by cells at the front edge of the cluster. “We don’t really know that much about them, other than they’re always at the front,” Hwang says. “So we call them leader cells. Not super creative.”
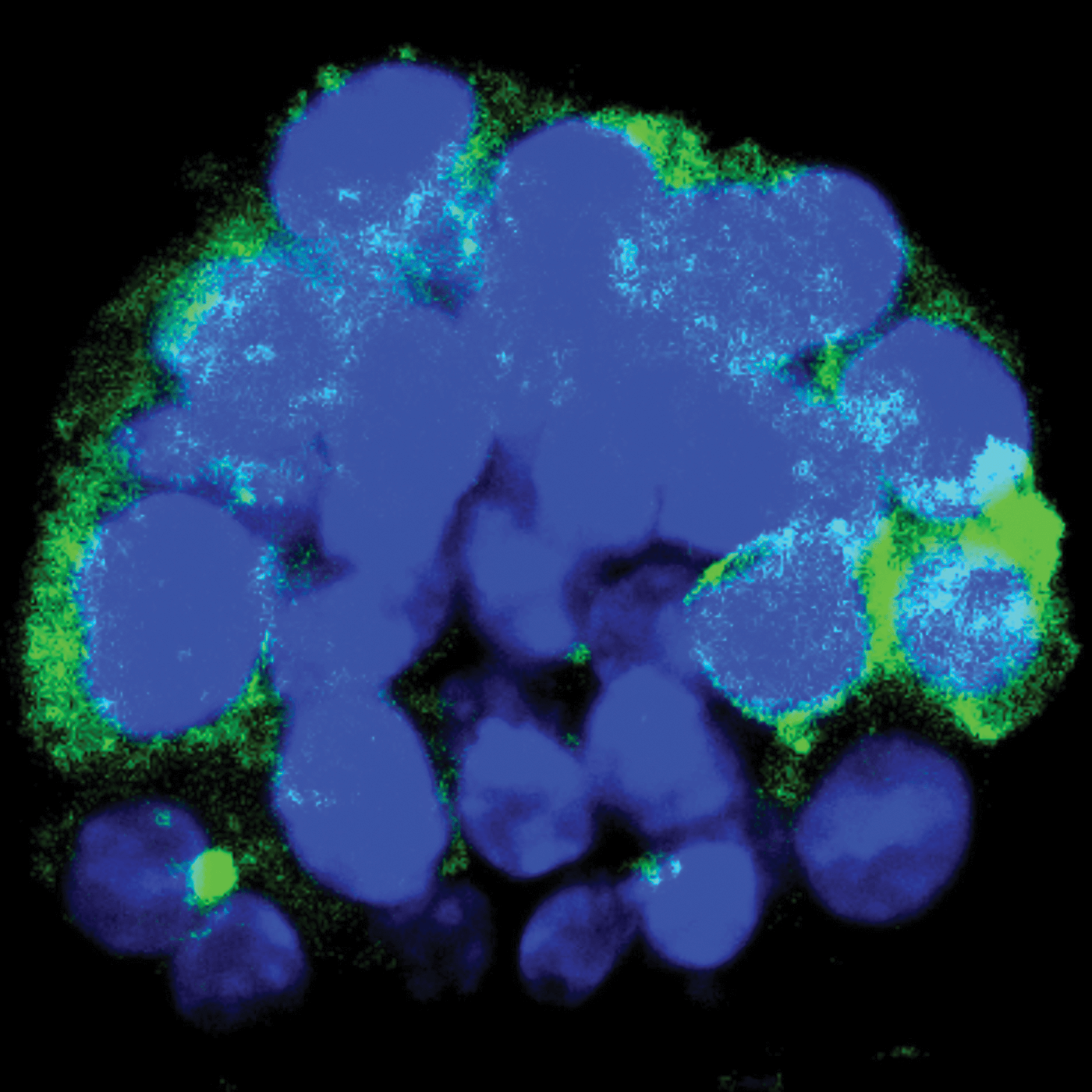
Leader cells are known to share a specific gene that’s turned on. A few years ago, to learn more about the behavior of these still-mysterious cells, Hwang and her colleagues ran an experiment in which they stained green any cell expressing that gene, using a fluorescent chemical to make them easier to spot. They saw the leader cells were at the edge of the tumor cluster, as originally suspected, but also sprinkled throughout.
Then the researchers mimicked the body’s signals to start migration — using a new method, which was a feat in itself — and watched as the leader cells, glowing green, pushed their way to the edge of the group to take the helm. It was all a bit unexpected.
“That was super exciting,” Hwang says of the findings reported in the journal Cancer Research in 2019 with colleagues at Washington University, where she was a postdoctoral researcher. “It’s not just any cell [at the front] that receives signals. These are kind of predestined or predetermined cells that actually move to that front edge and then lead migration. … I had never considered that these cells could just be anywhere.”
Bolstered by a five-year grant from the National Science Foundation, Hwang is trying to pinpoint the chemical and mechanical cues that trigger and drive a migration of breast cancer cells. (Jud Froelich)
The strength of that paper and of the ideas that followed led to the NSF early-career researcher grant she was awarded last year. For the 36-year-old Hwang, who arrived at VCU in fall 2019, the validation and financial cushion of the five-year, nearly $580,000 award have given her a runway to get her own lab and research agenda off the ground.
Most of the work uses breast cancer cells from mice and takes place inside transparent polymer gels, each roughly the size of a quarter and etched with a Lilliputian network of canals. Master designs for these “labs-on-a-chip” are fabricated on silicon in a clean room, similar to how computer chips are made, and then can be replicated in innumerable gels for experiments. In Hwang’s lab — with four doctoral students, four undergrads and a high school student — there are at any time dozens of these gels in play, each a cellular terrarium smushed onto a microscope slide.
In the gels, Hwang’s team aims to replicate the surroundings and nuances of a tumor’s world, which then can be watched and imaged in real time under a $200,000 microscope. It’s like “The Truman Show,” but for breast cancer. Building up from the collagen that largely comprises breast tissue, they can simulate dynamics like tissue stiffness, the velocity and direction of fluid through the body, and the naturally occurring waves of chemicals, while adding nutrients to keep the tumor cells alive. All of this can be connected to tiny pumps, allowing experiments to run for weeks, perhaps even months, if needed.
Through these lenses, Hwang’s lab is trying to pinpoint the chemical and mechanical cues — perhaps even from the way cells push against one another — that trigger and drive a migration. They’ve also been analyzing leader cells one by one to understand their similarities and how they pick up signals from the body.
In a January article in the high-profile journal Developmental Cell, Hwang and her collaborators found that even among leader cells there are different populations, with some seemingly better equipped to lead. The team also confirmed that the follower cells in a migrating breast cancer cluster are a mix of cell types, not merely a homogenous clump.
“They all have different signaling mechanisms, and probably they’re working with each other in different ways that might not be just migration,” Hwang says. “It might be other functions that we don’t know about.”
The situation is, once again, made “more complex,” she says, “more exciting.”
Historically, research on cancer’s spread has focused on tumor cells that migrate individually, Hwang says, and that’s led her and others to wonder whether some existing therapies might be less effective against the group dynamics of a cluster. So each new detail they reveal becomes a potential foil. Hwang also thinks her lab-on-a-chip designs someday could be used to test cancer drugs against an individual’s own tumor cells, for breast cancer or any cancer. “That’s kind of our pie-in-the-sky idea,” she says. “It could cut down on using the patient as the experimental platform, so to speak,” an alternative to moving down a best-guess list of drugs “and just kind of crossing your fingers.”
Hwang says she doesn’t feel burdened by a sense that the odds are piled against her. She sees in the microscope not a foe but a fascination.
The fight with cancer can seem like one that’s impossible for either side to prevail. There are trillions of cells in the human body, and it all can be taken down by a few jerk cancer cells ruining it for everyone. They grow without being asked; they ignore the usual cues that order a cell to stop growing or to just go ahead and die (a process called apoptosis); and they can co-opt other cells, even immune cells, into the effort.
On the other hand, cancer spreads through a process that’s known, clinically (and evocatively), as a “metastatic cascade.” A gauntlet of things have to go right for a primary tumor to form a second tumor elsewhere — from cleaving off to safely transiting to landing at a hospitable destination. By some estimates, fewer than 1 in 10,000 of the tumor cells that are capable of this journey actually succeed.
Those seem like decent odds for humanity — and yet, here we are: This year the American Cancer Society expects more than 600,000 people in the U.S. will die of cancer. Nearly 2 million people will be newly diagnosed, most commonly with breast cancer among women and prostate cancer among men.
Hwang says she doesn’t feel burdened by a sense that the odds are piled against her. She sees in the microscope not a foe but a fascination. “It sucks that it’s cancer,” she says, but from a scientific perspective, “it’s like a black box and we’re discovering things about it. … There’s still so much to be learned.” If anything, she says, “after this paper [published in January], I was more energized to get back in the lab because it’s like, ‘Oh, there’s all of these things for us to do.’”
The work could also apply to conditions besides cancer, she says. Much of back pain, for instance, is caused by disintegration of the cushioning between spinal discs — essentially, cell clusters that are disappearing instead of stubbornly sticking around.
And it’s been that cumulative potential of basic science — exposing detail, bit by bit, to form an increasingly complete picture — that got her interested in the subject and has sustained its pull.
Hwang had been studying biomedical engineering as an undergrad at Duke University when one of her best friends, a childhood leukemia survivor, invited her to help at a sleep-away summer camp his family started for kids with cancer and blood disorders being treated at Nicklaus Children’s Hospital in Miami. That was about 15 years ago, and Hwang still leaves the lab for a week each summer to work at the camp, a shift that draws her toward “a group of people who are basically family,” many of whom are former campers; toward traces of cancer you don’t need a microscope to see. In faces old and new, she finds a reminder of how much of the black box has been illuminated, and of all that remains in the dark.
